|
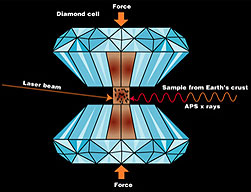
While growing up in Northern California, I developed a deep interest in wine and wine making. While I always had a passion for music and being a musician, I also loved just about everything else academic, in particular languages and the sciences. I became involved with a small group of friends who also loved wine and we began to buy grapes and produce wine. In winemaking there are various decisions in that process that are guided by the particularly chemistry of that wine. As the member of the group with the highest proclivity towards science, I began to study and understand this chemistry and the techniques of chemistry so that they might be applied. I had begun college in the Jazz Studies program under the remarkable Dwight Cannon. Dwight was an inspiring trumpet player who brought refreshing ideas of improvisation to the academic program. We would often have improvisation classes where you were not allowed to play your own instrument or just use your voice or the music stands. I decided to transfer to the University of California at Santa Cruz and ended up (via majors in music, classical languages and geology) graduating with a degree in chemistry. I decided to continue at Cornell University where I had the chance to take graduate courses with the deeply inspiring astronomer-chemist-thinker Carl Sagan and with whom I nearly opted to do my doctoral thesis. I began however to work with the brilliant geophysicist William Bassett who worked with something called the Diamond Anvil Cell:
This is essentially two gem quality diamonds with the points ground to a small face. Opposing forces are applied generating up to 3,000,000 atm (we live at a pressure of around 1 atm). Since diamonds are transparent, one can also focus a laser on the sample in the cell and generate very high temperatures thus simulating conditions deep inside the mantle of the earth. We were working on the understanding the behavior of the element Carbon by placing small amounts of graphite in the cell and observing their behavior. I had noticed that the laser we were using was not working as well as it should. I had made some adjustments to the optical elements but began an experiment still running the laser at very high powers. When I began to run the laser across the face of the diamond incredible amounts of light was produced from the heating of the diamond face and a small TV camera used to monitor the experiment began to smoke. It was quite dramatic and I thought that perhaps I had destroyed some very expensive equipment and that my graduate career was over! My advisor was frankly more interested in returning to the initial experiments as we were preparing for a conference. I retained the diamonds and quietly began to study them under the electron microscope. It became clear that something very interesting had occurred and that indeed the face in contact with the sample at high pressure had a “furrow” due to melting. For details please download the paper "Melting of a Diamond" from the scientific journal Science. This was a classic case of serendipity in science! |